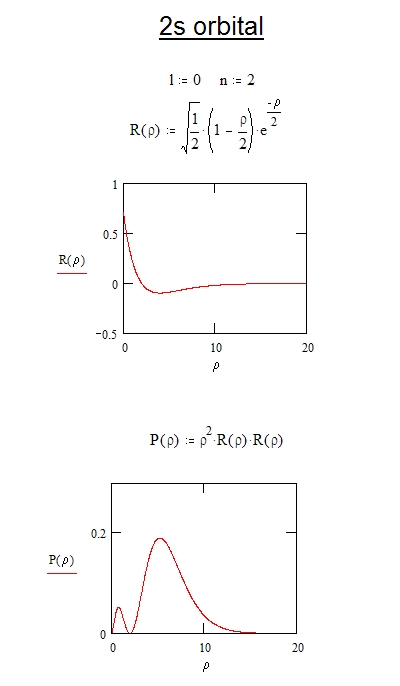
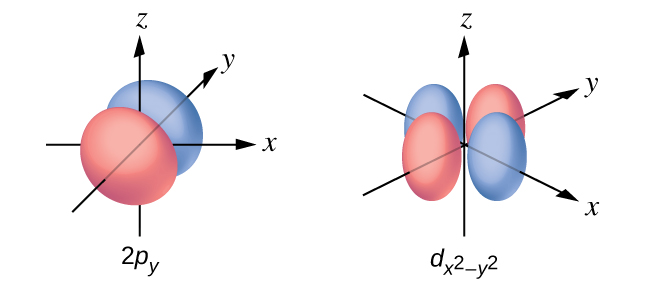
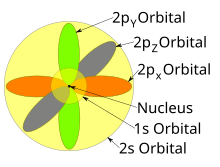
X2 Y2 Orbital
This is the easy way:.

X2 y2 orbital. 1 2 = 1, 2 2 = 4, 3 2 = 9. The number of orbitals in a shell is the square of the principal quantum number:. This is the hard way:.
N = 3, l = 2. The importance of the orbital degree of freedom is pointed out as well as the charge and spin ones. The shape and size of an orbital can be determined from the square of the wave function.
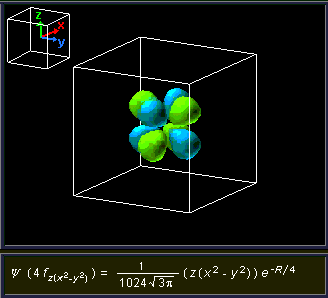
4f z(x 2-y 2) 4f x(x 2-3y 2) n = 5 and beyond;. N = 5 and beyond;. Be sure to show and label the axes.
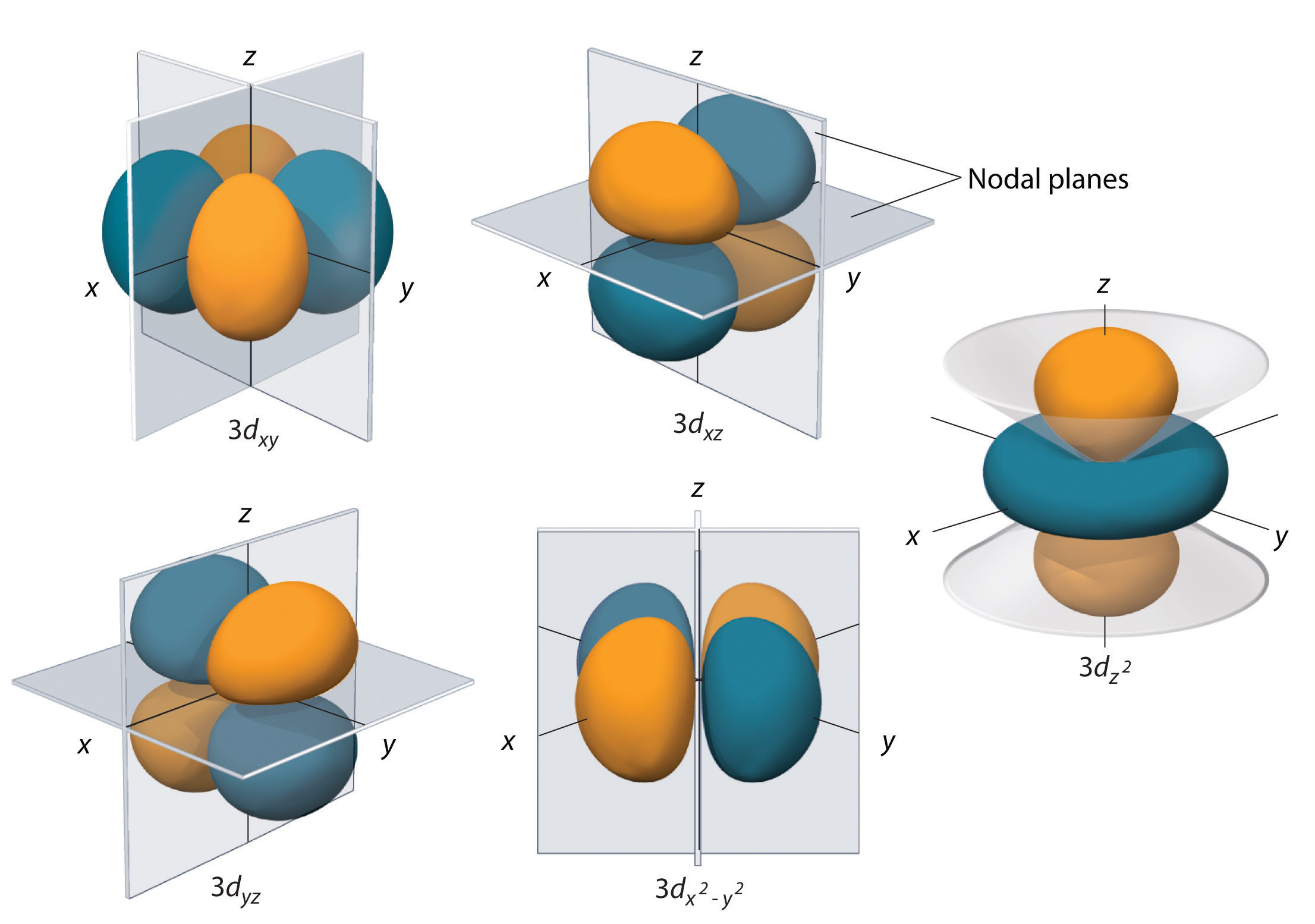
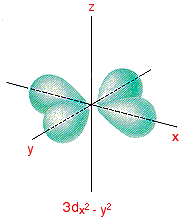
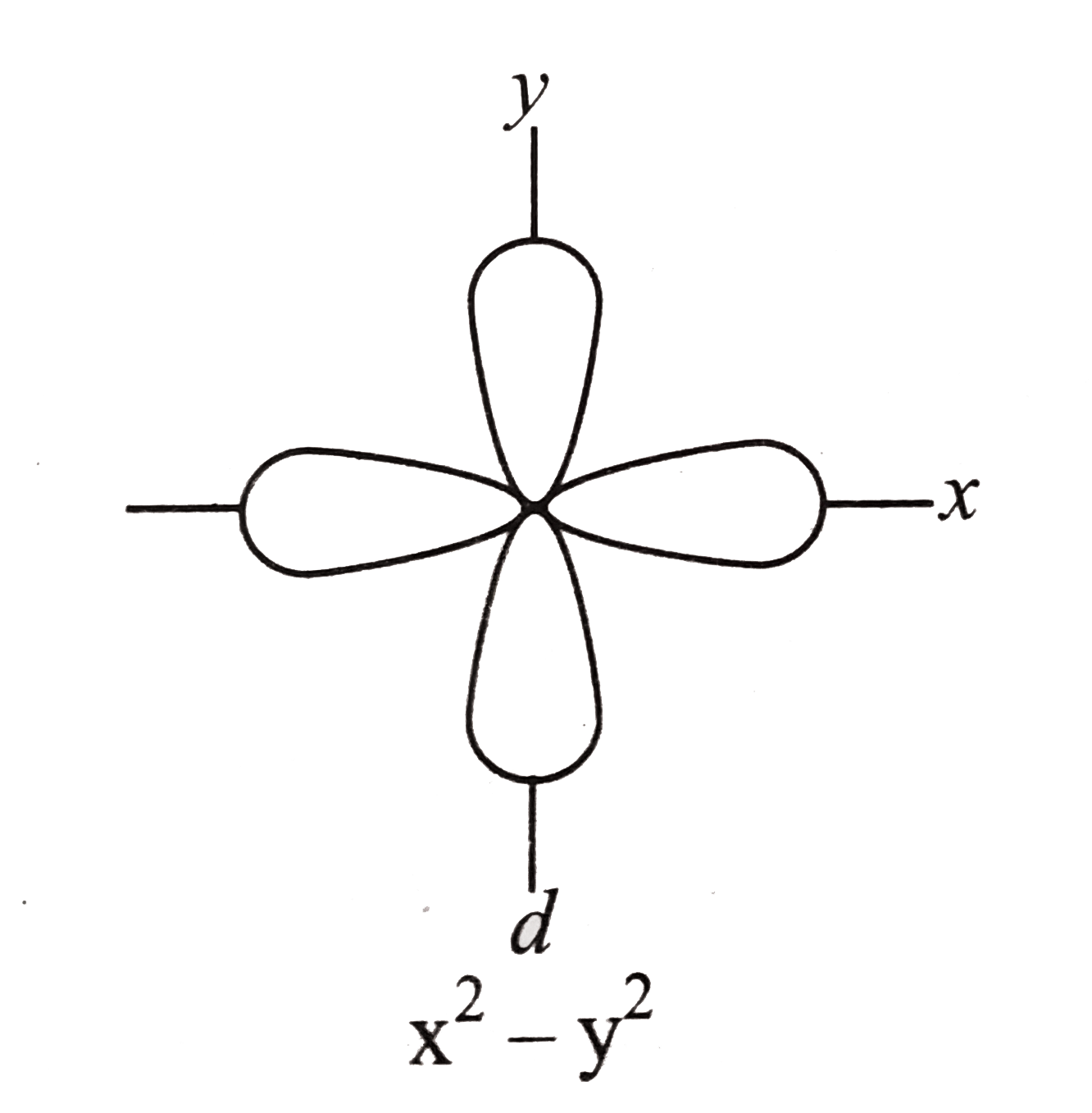
Out of these five d orbitals, shapes of the first four d-orbitals are similar to each other, which is different from the d z 2 orbital whereas the energy of all five d orbitals is the same. 3d z 2 orbital:. In the d x 2-y 2 orbital, one pair of lobes lies on the x axis and the other lies on the y axis.
X The coordinate (x, y, and z) axes are also shown. The angular wave function for the hydrogen atomic 3d_(x^2-y^2) orbital ((l,m_l) = (2,2)) is given. The d z 2 orbital looks very different from the other four:.
Bisecting the xy axes. It looks like a \(2p_z\) orbital combined with an additional doughnut of electron probability lying in the xy plane. Suppose an atom with its nucleus at the origin has an electron in a 3d 2 2 orbital.
N = 4 shell;. N = 2 shell;. The Chime plugin (Version 2.0 or higher) is required to view this page.
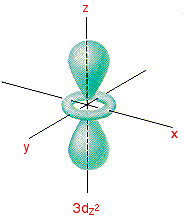
This 3D model was originally created with Sketchup 7 and then converted to all other 3D formats. For math, science, nutrition, history. The d z 2 orbital has a somewhat novel configuration, in which two lobes lie on the z axis and are encircled by a doughnut-shaped orbital centred on the z axis.
This orbital is a bit like a p orbital with a Saturn-like-ring around its middle. 5) none of the. Would increase by 2.
3d x 2-y 2;. This orbital is in the xy-plane. Enter z (pm) Contributors.
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals into new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory.Hybrid orbitals are very useful in the explanation of molecular geometry and atomic bonding properties and are. Dx2, dxy, dxz, dzx, d(x2-y2) - Orbital Model Sets - Kit of 1:. Orbitals are the regions of space in which electrons are most likely to be found.
The 3d_(x^2-y^2) orbital has lobes that point along the x and y axes:. 0606K + pkg - Set of 5 d Orbitals:. The 6g z 2 xy an abbreviation for 6g xy(6z 2 - x 2 - y 2) and 6g z 2 (x 2 - y 2) an abbreviation for 6g (x 2 - y 2)(6z 2 - x 2 - y 2) orbitals (middle row in the image above) each have twelve lobes and are related to each other by a 45° rotation about the z-axis.
As found previously,23 it is thus the axial orbital that encodes the ma-terials dependence. This is the \(3d_{x^2−y^2}\) orbital. The symbols used contained in right here are:.
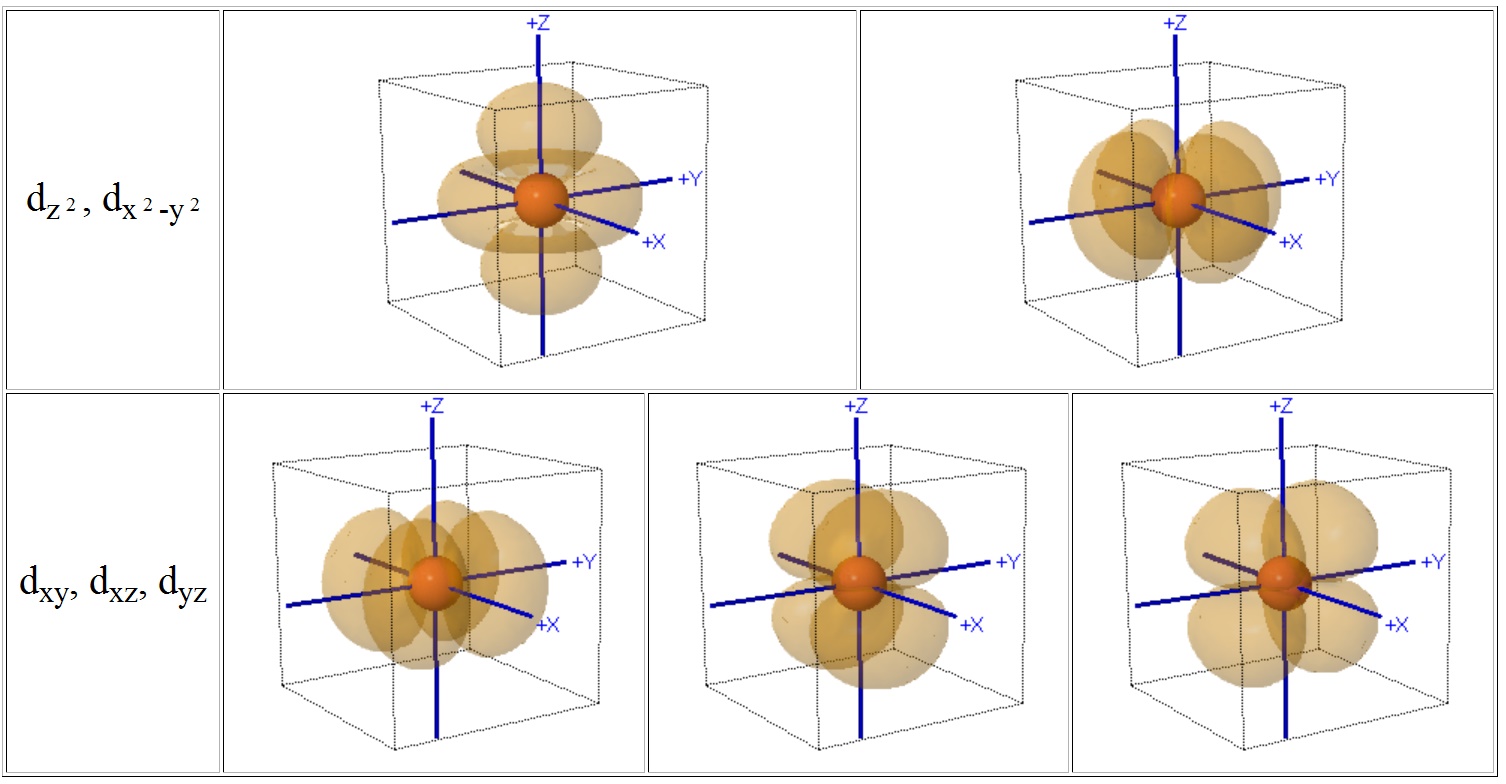
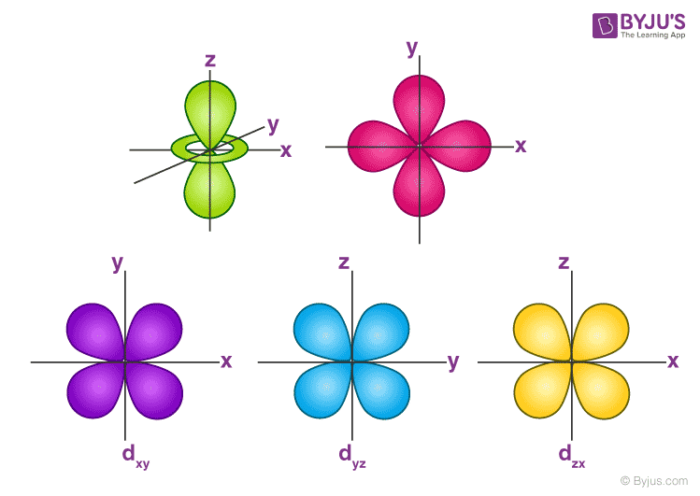
Even though the d z 2 orbital looks different, it has the same energy as the other four d orbitals. You can select an orbital to view by clicking on the appropriate button. The d xy , d yz and d zx orbitals have same shape i.e., clover leaf shape but they lie in XY, YZ and ZX- planes respectively.The d z2 orbital is symmetrical about Z-axis and has a dumb - bell shape with a doughnut shaped electron cloud in the centre.
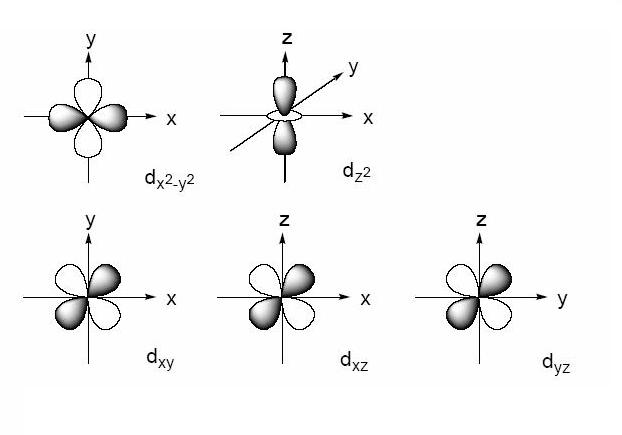
3) specifies the 3-D shape of the orbital. These orbitals are designated as d xy, d yz, d xz, d x 2 –y 2 and d z 2. Which of the above fourth shell orbitals is a 4 d x 2 y 2 orbital A orbital a B.
Likewise d z2 (along z) does not contact the negative charges. This sketch is about 1800 pm wide. This means that the populations of x2 –y2 and 3z 2 – r 2 orbitals in the e g state play a key role in understanding the transport and magnetic properties of this system.
As a result, the energy of this arrangement would be lower than that for the complex having O h symmetry. Therefore, the two nodal planes point in between the lobes, i.e. This is a continuous function, thus nodal region must have null value of wave function.
How would the dx2?y2 orbital in the n=5 shell compare to the dx2?y2 orbital in the n=3 subshell?. Be sure to show and label the axes. The principal quantum number (n):.
The only difference between these two orbitals is that the d_(x^2-y^2) lobes are along the axes and the d_(xy) is rotated 45^@ counterclockwise. X2-y2 orbital which forms an antibonding combination of Ni x2-y2 and O p x/p y is identical in three compounds. Orbital will have more contact with the negative charges than the d x2-y2 orbital (no charges point at the d x2-y2 orbital but the d xy orbital is between the axes).
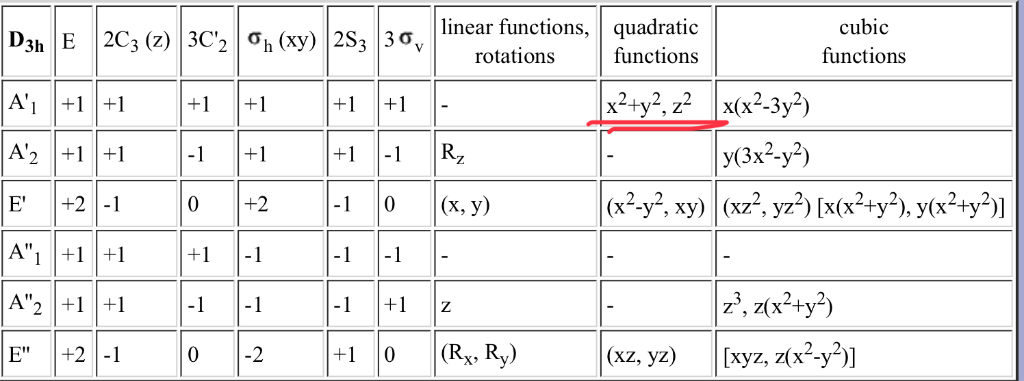
Which of the above fourth shell orbitals is a 4 d x 2. The number denotes the energy level of the electron in the orbital. Orbital Theory Transformational properties of atomic orbitals Atomic orbital Transforms as s x2+y 2+z 2 px x py y pz z dz2 z2, 2z 2-x2-y2 dx2-y2 x2-y2 dxy xy dxz xz dyz yz S py • When bonds are formed, atomic orbitals combine according to their symmetry.
Ratings 92% (13) 12 out of 13 people found this document helpful. Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For the 3d yz orbital lies in the plane defined by the y and z axes, and between the y and z axes, for the 3d x 2-y 2 orbital lies in the plane defined by the x and y axes, and directly along the x and y axes, and:.
Light with helical phase fronts is associated with orbital angular momentum (OAM) states denoted as φ (r, ϕ) = exp (i l ϕ), where ϕ is the angular coordinate and l can take any integer value. The m_{l} value would be the same. N = 3 shell;.
B).The value of ?. However, two of the electrons in the d x 2 − y 2 and d z 2 orbitals would occupy the d z 2 orbital, whereas only one would be in the higher energy d x 2 − y 2 orbital. 3d x 2-y 2 orbital:.
The orientation of the orbital would be rotated 45 degrees along the xy plane. The calculation of the 40,000 points comprising the image (0x0 pixels) is quite a chore for the computer so be patient if you are working on an older machine!. D x2-y2 d xy d z2 e set (stabilized) d yz t 2 set d xz (destabilized) z x y.
You can view more similar questions or ask a new question. The fourth orbital in this subshell lies along the X and Y axes and is called the 3d x 2-y 2 orbital. Chem 59-250 Symmetry of orbitals and functions yz y, R x 1-1-1 1 B 2 xz xy x 2,y 2,z 2 x, R y R z z-1 1-1 1 B 1-1-1 1 1 A 2 1 1 1 1 A 1 σ ’ v (yz) σ v (xz) C 2 E C 2V z y x z y x A p z orbital has the same symmetry as an arrow pointing along the z-axis.
R = radius expressed in atomic gadgets (a million Bohr radius = fifty two.9 pm) ?. The lobes are alternating phase going around the orbital. 4) specifies the maximum number of electrons.
Expanded View of the 3d x 2-y 2 Orbital. Most likely, your textbook will include a discussion of the derivation for at least the octahedral case. Now, of course this would be very difficult if you don't know the functional form of the two orbitals, and even if you did know the functional form, evaluating the integral would still be messy.
There are rigorous mathematical ways to deduce the symmetry labels of d-orbitals (or any orbital, for that matter) under an environment of a certain symmetry, as provided by point group theory, which involve symmetry tables and so on. You can rotate the sketch for a better view of the orbital by dragging the slider with your mouse. I'm just absolutely lost.
On the other hand, while the features of axial orbital is same between NdNiO 2 and PrNiO 2, it is different in LaNiO 2. N = 4 shell;. 1) specifies the subshell of the orbital.
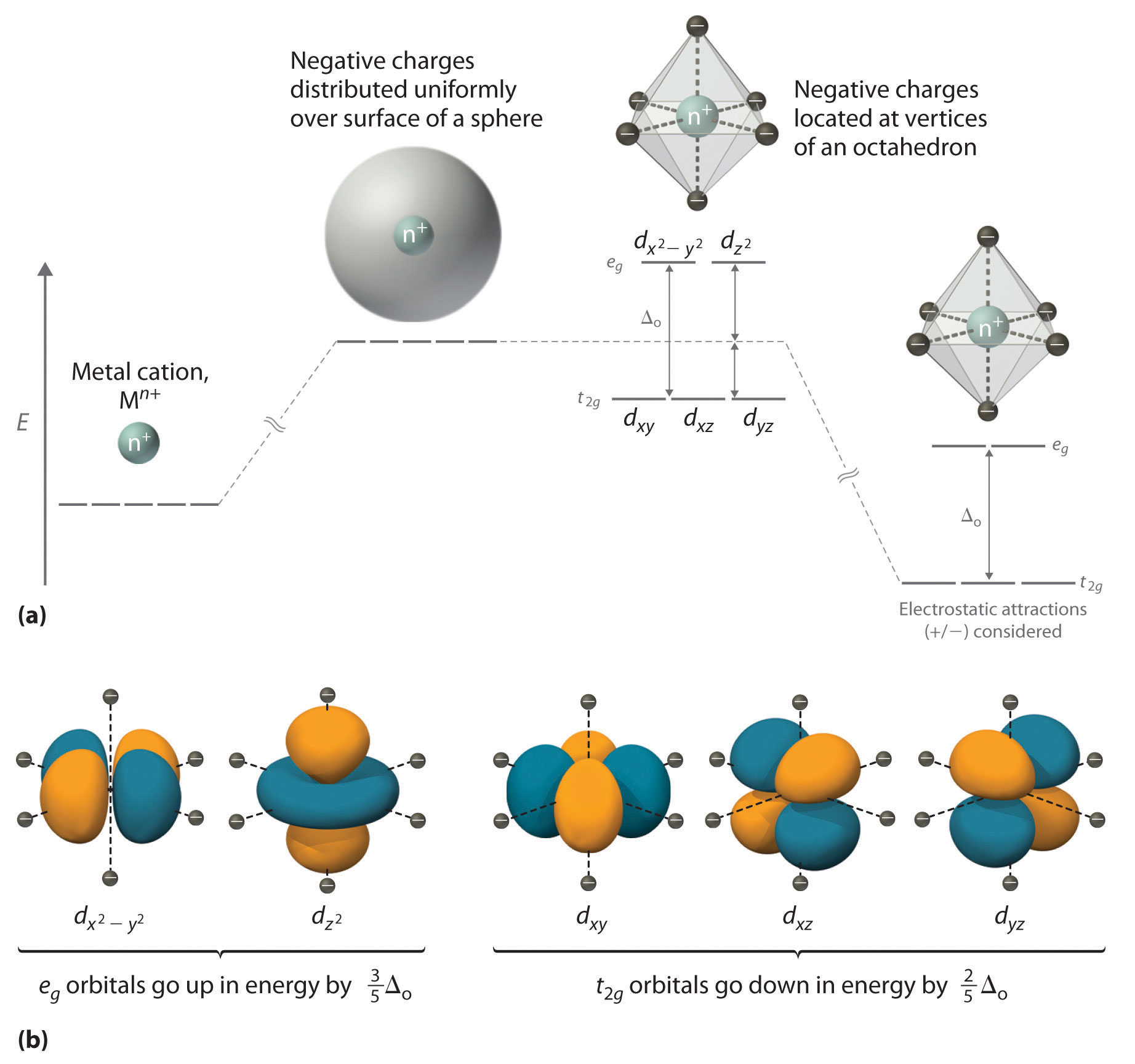
In an uncomplexed metal ion in the gas phase, the electrons are distributed among the five d orbitals in accord with Hund's rule because the orbitals all have the same energy. Woerdman, “ Orbital angular momentum of light and the transformation of Laguerre-Gaussian laser modes,” Phys. It is x^2 – y^2.
= 2Zr/n the place n is the critical quantum quantity (3 for the three-D orbitals) (Schrödinger wave equations) Radial wave function, R3d. Thus d orbital corresponds to 4 double dumb-belled shapes (d xy, d yz, d zx, d x 2 y 2) with the atomic nucleus at its centre and one dumb belled with dough nut shaped (d z 2). Here is a sketch of a 3d 2.
We have investigated the orbital state in La2-2xSr1+2xMn2O7 by. 4d x 2-y 2;. The splitting of the two sets of orbitals (e g and t 2g) is not equal.
Sketch the boundary surface of a dx2−y2 and a py orbital. X2−y2 state, with mass enhancement of ∼ 2.4. The sp, sp 2 and sp 3 Hybrid Orbitals.
What Is A Hybrid Orbital?. Now, to see if two orbitals overlap, in your case $\ce{p_y}$ and $\ce{d_{x^2-y^2}}$, one simply needs to compute the quantity defined above. This orbital's main lobes align with the z axis.
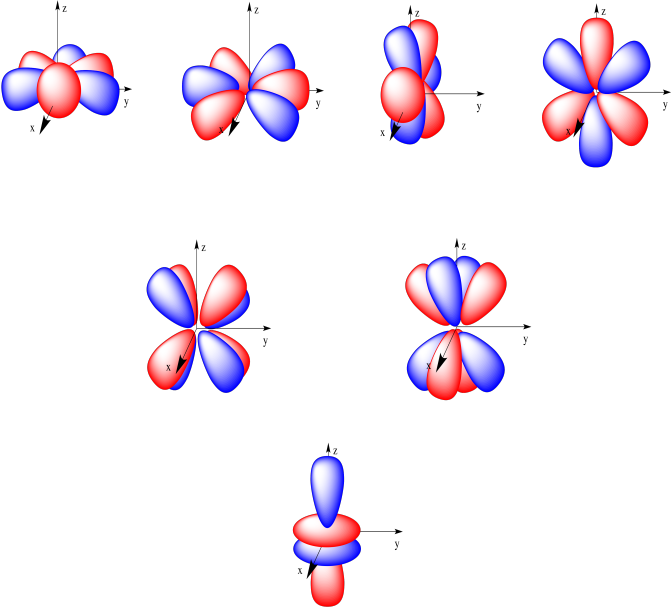
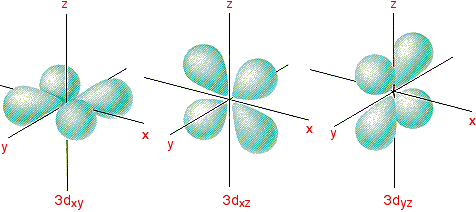
4f y(3x 2-y 2) 4f xyz;. For example, 3d xy, 3d yz, 3d zx, 3d x 2-y 2 and 3d z 2. The value of l would increase by 2.
School University of Ontario Institute of Technology;. 🤓 Based on our data, we think this question is relevant for Professor Altomare's class at UCF. Industrial & Scientific Skip to main content Try Prime.
N = 1 shell;. D x 2-y 2. For clarity, the ligands have been omitted from the d x 2 − y 2 d x 2 − y 2 orbital so that the axis labels could be shown.
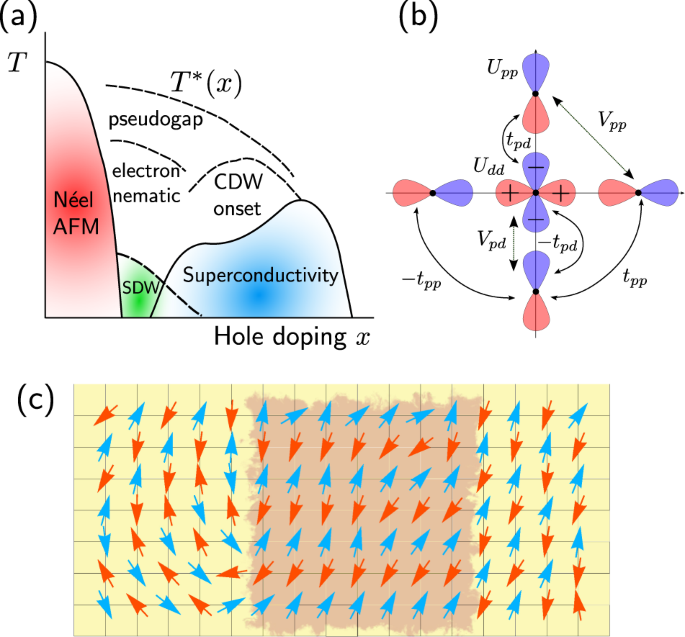
Most of the space occupied by the fifth orbital lies along the Z axis and this orbital is called the 3d z 2 orbital. The four lobes are aligned with the x and y axes. We believe these findings are helpful to understand the unconventional super-conductivity in this system.
This can be done rigorously or easily. C).The radial probability function would include two more nodes. The lobes of the d x 2 - y 2 orbital also lie in the xy plane, but the lobes lie along the x and y axes.
The angular part of mathd_{x^2-y^2}/math is pr. The contour of the orbital would extend further out along the x and y axes. A fourth d orbital has lobes lying along the x and y axes;.
For the 3d z 2 orbital lies directly along the z axis. Course Title CHEM 1800U;. For instance, the corre-lated Ni-3d x2−y2 orbital is most relevant for electron pairing, and the change of topology of Fermi surface.
X-axis = yellow y-axis = green z-axis = blue:. = 3. approximately e = 2.718 approximately Z = effectual nuclear fee for that orbital in that atom. The fifth 3d orbital, called the \(3d_{z^2}\) orbital, has a unique shape:.
(x 2 −y 2) f x(x 2 −3y 2) f y(3x 2 −y 2) n = 1:. The radial probability function would include two more nodes. The term atomic orbital may also refer to the physical region or space where the electron can be calculated to be present, as predicted by the particular mathematical form of the orbital.
A).The contour of the orbital would extend further out along the x and y axes. Each orbital is denoted by a number and a letter. It is quickly suppressed to ∼ 2.1 upon heavy hole-doping.
The d_(x^2-y^2) has two vertical nodal planes bisecting the x and y axes, and the d_(xy) has an bb(xz) and bb(yz) nodal plane. • Symmetry properties and degeneracy of orbitals and bonds can be learned. The doughnut is bisected through its circumference by the plane formed by the x and y axes.
Hence p orbitals have three orientations in space. Nodal region is just where a wave function is changing its signal (from positive to negative or vice versa). D zx, d x 2-y 2 and d z 2;.
2) specifies the principal shell of the orbital. Native format is .skp 3dsmax scene is 3ds Max 16 version, rendered with Vray 3.00 d x2y2 orbital superimosed on an octahedral model. Stack Exchange network consists of 176 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers.
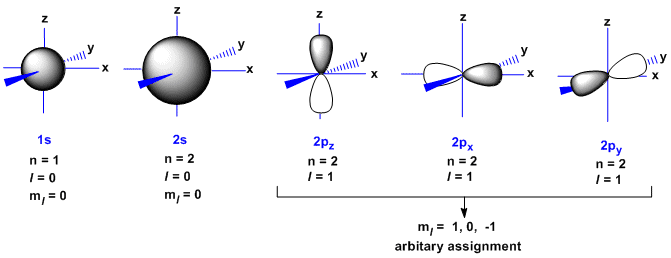
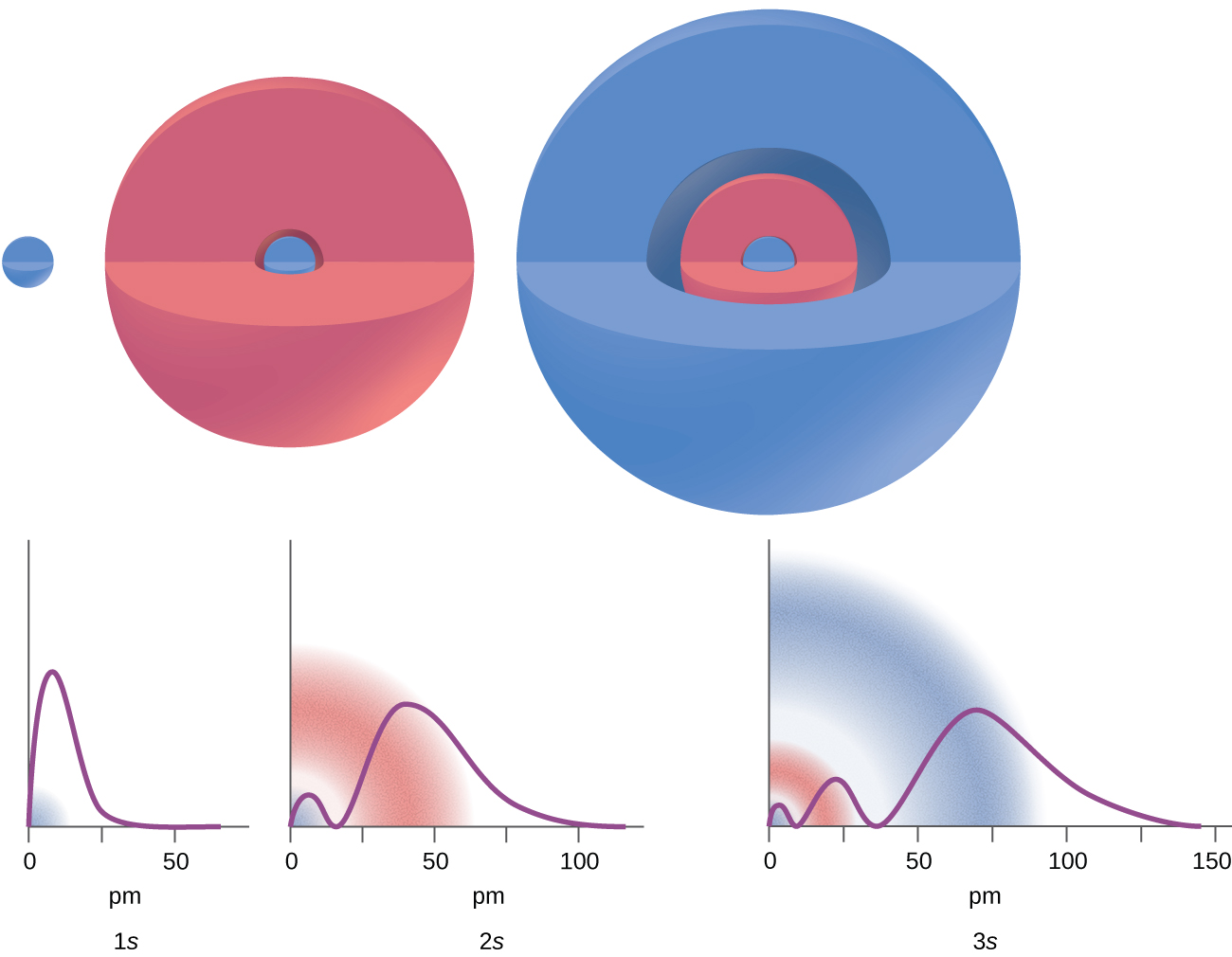
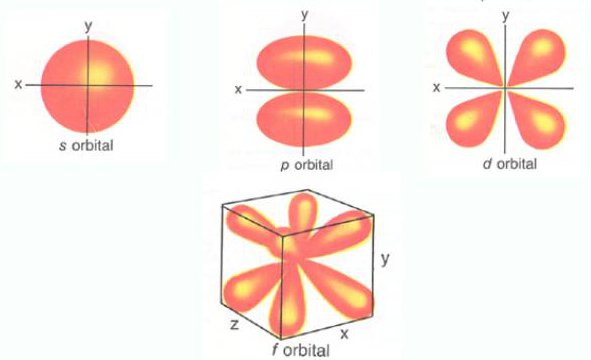
For p orbital Azimuthal quantum number l = 1 and the magnetic quantum number m = -1, 0, +1. 2 refers to the next energy level further out, and so on. Thus 1 refers to the energy level closest to the nucleus;.
3d x 2-y 2 orbital:. It has two lobes along the z axis and a "doughnut" in the xy plane.

Quantum Numbers

A Copper D X 2 Y 2 Orbital And Its Surrounding Oxygen P X Or P Y Download Scientific Diagram

Solved What Is The Symmetry Label For A D X 2 Y 2 Orbit Chegg Com
X2 Y2 Orbital のギャラリー

The Shape Of The 4d Orbital Chemistry X Youtube
Http Casey Brown Edu Chemistry Research Lswang Publications 360 Pdf

Is It 3dx2y2 Or 3dx2 Y2 Chemistry Structure Of Atom Meritnation Com
Atomic Orbital
Q Tbn 3aand9gctt Z3b6pn9vyt3gk4cgjc5ixxiwfobiceiv Cew Cv9kv6zmmi Usqp Cau

Atomic Orbital Chemistrygod
6 6 3d Representation Of Orbitals Chemistry Libretexts

File Fz X2 Y2 Orbital Png Wikimedia Commons

The D X 2 Y 2 Orbitals Of Fermions On Copper Ions And Px And Py Download Scientific Diagram

Introduction To Orbitals Stahl 9 12

The Roles Of 4f And 5f Orbitals In Bonding A Magnetochemical Crystal Field Density Functional Theory And Multi Reference Wavefunction Study Dalton Transactions Rsc Publishing Doi 10 1039 C6dte

Hybrid Orbitals

Science Skool Atomic Orbitals

Figure 4 Orbital Ordering Scenarios For Jahn Teller Active 3 I D I Sup 9 Sup And 4 I D I Sup 9 Sup Layered Perovskites
Structure Reactivity Atoms

Chemistry D Orbitals
Q Tbn 3aand9gcseyobbyyxbjn6tvzukgg8pqxexr4l9yuq G9cwemw2k9xszngu Usqp Cau
2 8 Bonding In Octahedral Complex Ions Crystal Field Theory Chemistry Libretexts

How Are D Orbitals Named Dz Dxy Dyz Dxz And Dx Y Quora

How Are D Orbitals Named Dz Dxy Dyz Dxz And Dx Y Quora
Q Tbn 3aand9gcqkmfnyiafrl15xmtugyzntdsh3bes4t5 Mafe1zuzdwzpff2xm Usqp Cau

How Is The 5 Fold Degeneracy Of Atomic D Orbitals Removed In The Case Of Tetrahedral Compounds And Square Planar Compounds Study Com

3dx2 Y2x Mov
.png)
How Do I Determine T Questioncove
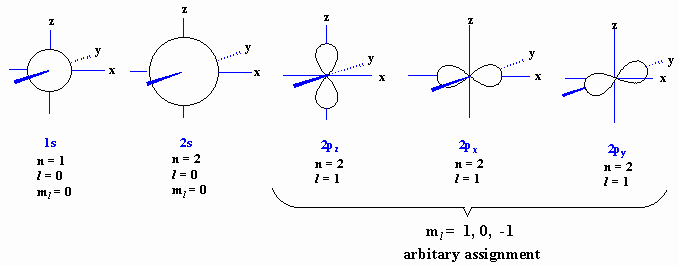
Ch 1 Electrons And Orbitals

Oneclass Consider The Orbitals Shown Here In Outline A What Is The Maximum Number Of Electrons Co

Quantum Numbers A Short Tutorial Bohr Model Of Hydrogen Atom An E S Is Found In Specific Energy Levels These Levels Represent A Fixed Distance From Ppt Download
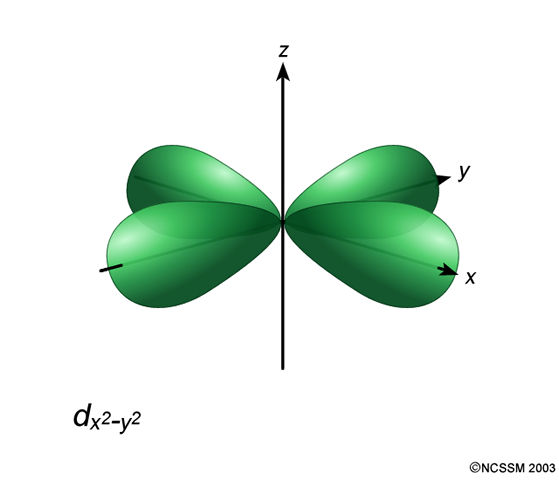
What Is The Significance Of The 3d X 2 Y 2 Atomic Orbital Socratic
Spectroscopic And Magnetic Properties Of Coordination Compounds Chemistry Atoms First Openstax Cnx

Chapter 2 Section 2 4 Open Inorganic Chemistry
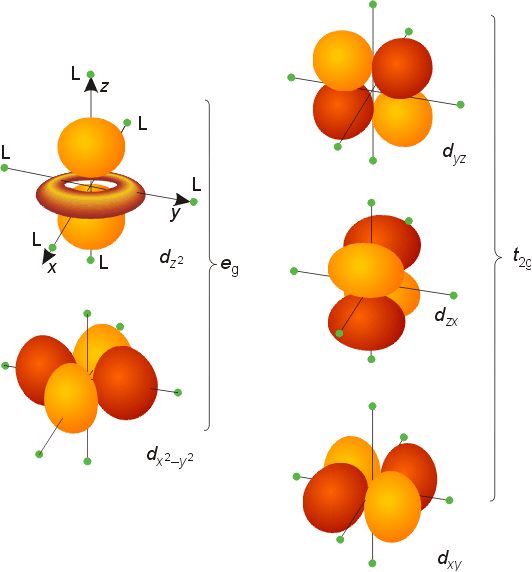
D Metal Complexes
Http Nanowires Berkeley Edu Teaching 104a 1402 Pdf
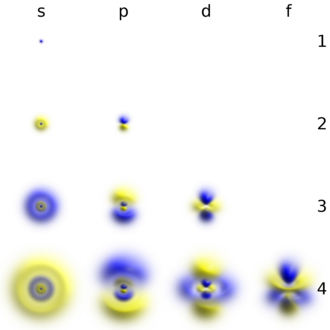
Atomic Orbital Wikipedia

A The Five D Orbitals Dz2 Dx2y2 Dxy Dxy Dyz On The Central Transition Metal Of Complex Ions Not Used To Form Covalent Bonds To The Ligands Have The Same Energy Prior To
Onlinelibrary Wiley Com Doi Pdf 10 1002 Ange

Magnetic Quantum Number Chemistrygod

Total Dos In Fm Magnetic And X 2 Z 2 Y 2 Z 2 Orbital Orderings Download Scientific Diagram

Chapter 2 Section 2 4 Open Inorganic Chemistry
What Are The Nodal Planes Of The D X 2 Y 2 Orbital Socratic
Crystal Field Theory Cft

What Is The Maximum Number Of Electrons That Can Be Present In A Single 3d Orbital Quora
D X2y2 Orbital Superimosed On An Octahedral Model 3d Warehouse

Shape Of The Antibonding T 2g Xy And E G X 2 Y 2 Orbitals Of Download Scientific Diagram
Solved Generate D Orbital Splitting Diagrams For The Foll Chegg Com
Q Tbn 3aand9gcqqfx6pzmsxdi2uw9vzetjpauxulquptk5uk642sgjdu4qcc5vw Usqp Cau
Enhanced Nematic Fluctuations Near An Antiferromagnetic Mott Insulator And Possible Application To High T C Cuprates Npj Quantum Materials

Orbitals Definition Types Orbital Shapes Quantum Numbers

What Is The Difference Between Dx2 Y2and Dz2 Orbitals Quora
The Electron Density In The Xy Plane In 3d X 2 Y 2 Orbital Is
What Kinds Of Bonds Does The D X 2 Y 2 Orbital Form In Transition Metal Complexes Socratic

Chemistry The Central Science Chapter 6 Section 6

Crystal Field Theory Chemistry Libretexts
Complex Ions More About D Orbitals
Ch 1 Electrons And Orbitals

Orbit Subshell Orbital Electron
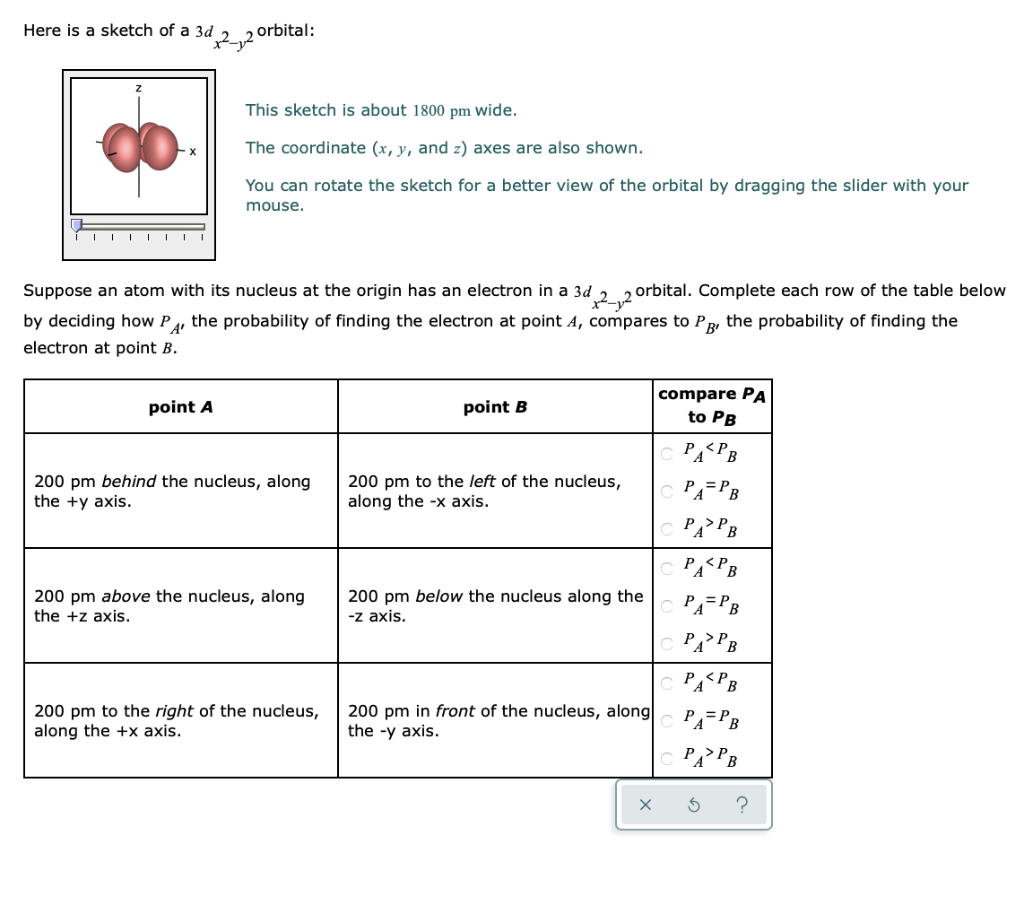
Solved Here Is A Sketch Of A 3d 2 2 Orbital This Sketch Chegg Com

Shapes Of Atomic Orbitals

Magnetic Quantum Number Chemistrygod
6 3 Development Of Quantum Theory Chemistry
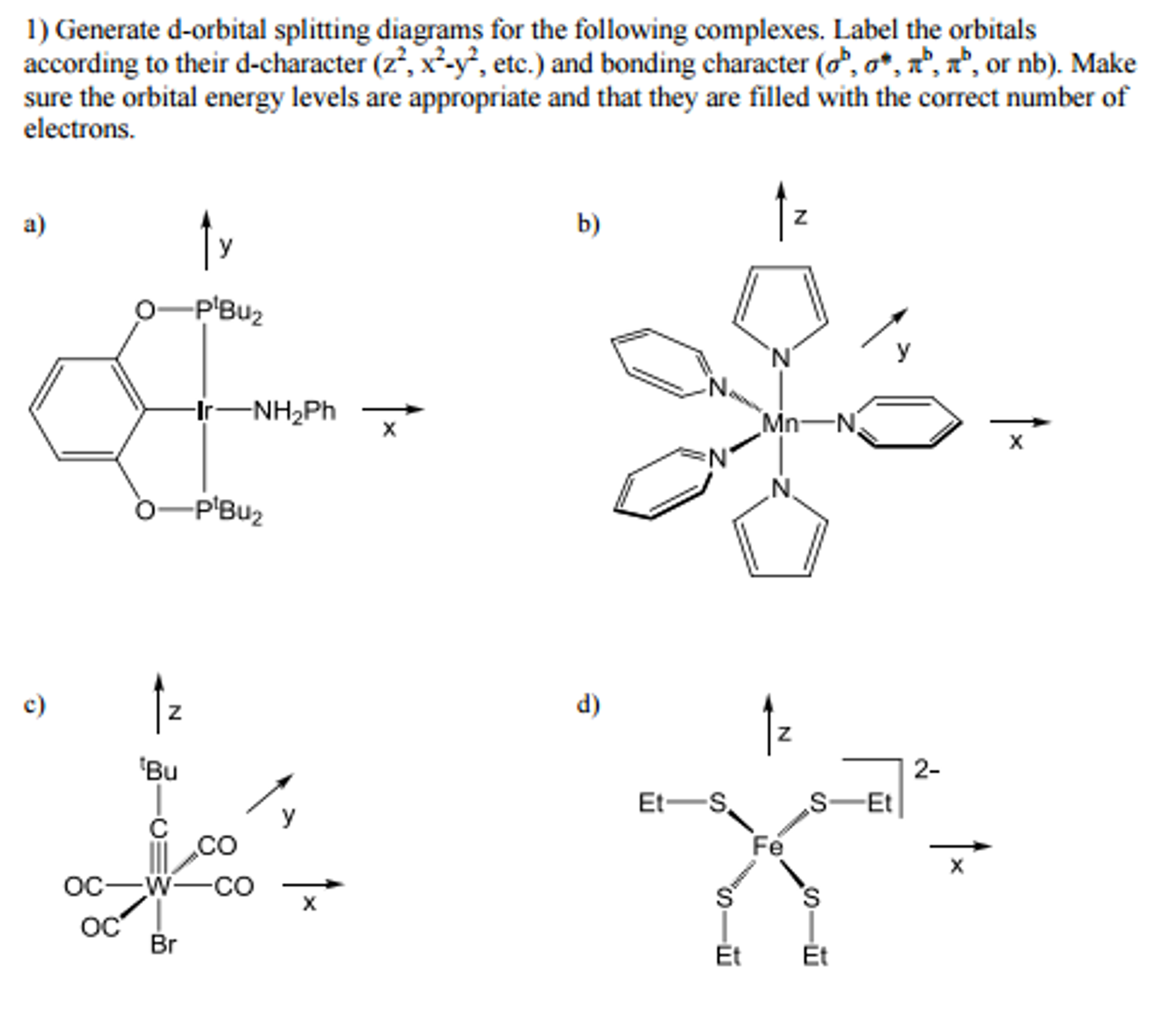
Generate D Orbital Splitting Diagrams For The Foll Chegg Com

Bonding In Hydrogen
Shapes Of Dorbitals Assignment Help Homework Help Online Tutoring Chemistry Help

Atomic Orbital

6 6 3d Representation Of Orbitals Chemistry Libretexts
Complex Ions More About D Orbitals

Which Group Of D Orbitals Has Greater Energy Quora
6 3 Development Of Quantum Theory Chemistry

File Single Electron Orbitals Fz X2 Y2 Jpg Wikimedia Commons

Atomic Orbital Chemistrygod

Orbital Symmetry And Orbital Excitations In High T C Superconductors

What Is The Difference Between Dx2 Y2and Dz2 Orbitals Quora

File Atomic Orbital Cloud N4 Fz X 2 Y 2 Png Wikimedia Commons

Geos 306 Fall 04 Lecture 2 The Nature Of The Atom
Visualizing Atomic Orbitals
Bond Orders Of The Diatomic Molecules Rsc Advances Rsc Publishing Doi 10 1039 C9rad

Bonding In Co Ordination Compounds Study Material For Iit Jee Askiitians
Orbit Subshell Orbital Electron

Chapter 2 Section 2 4 Open Inorganic Chemistry

Chapter 10 Coordination Chemistry Ii Bonding Pdf Free Download

3dx2 Y2x Mov
Atomic Orbital Wikipedia

State True Or False The Electron Density In Xy Plane Of 3dx 2 Y 2 Orbital Is Zero
Spectroscopic And Magnetic Properties Course Hero
Http W0 Rz Berlin Mpg De Imprs Cs Download Symmetry11 2 K Horn Pdf
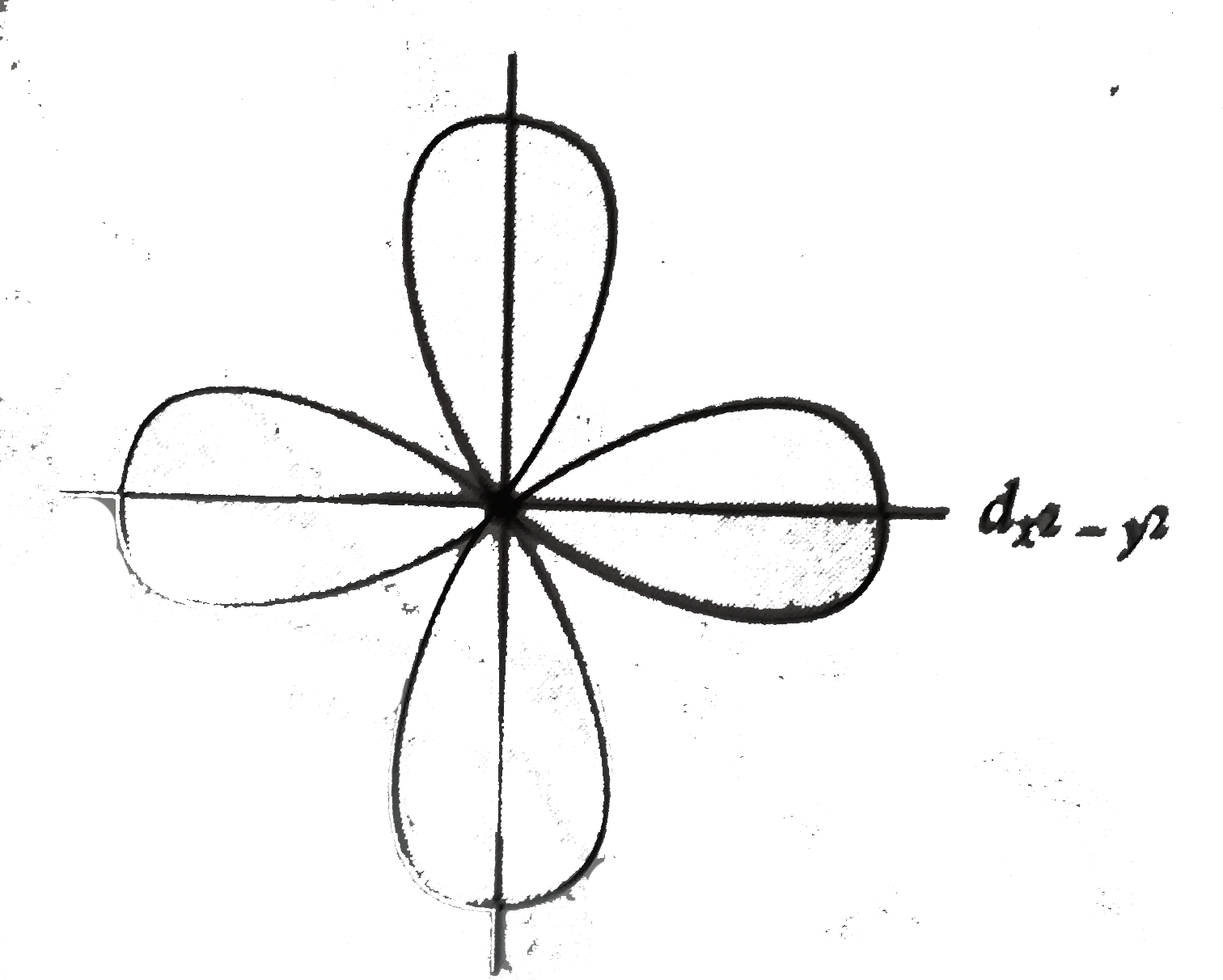
The Electron Density In The Xy Plane In 3d X 2 Y 2 Orbit
Orbitals Chemistry Shapes Of Atomic Orbitals Shape Of S P D And F Orbital
In The Character Table Which D Orbital Does X 2 Y Chegg Com
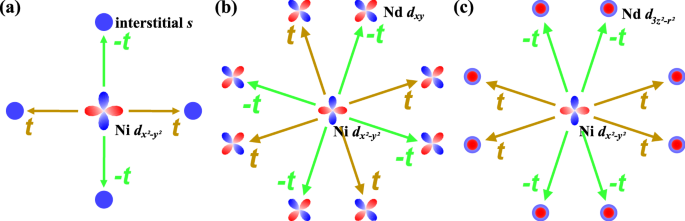
A Substantial Hybridization Between Correlated Ni D Orbital And Itinerant Electrons In Infinite Layer Nickelates Communications Physics
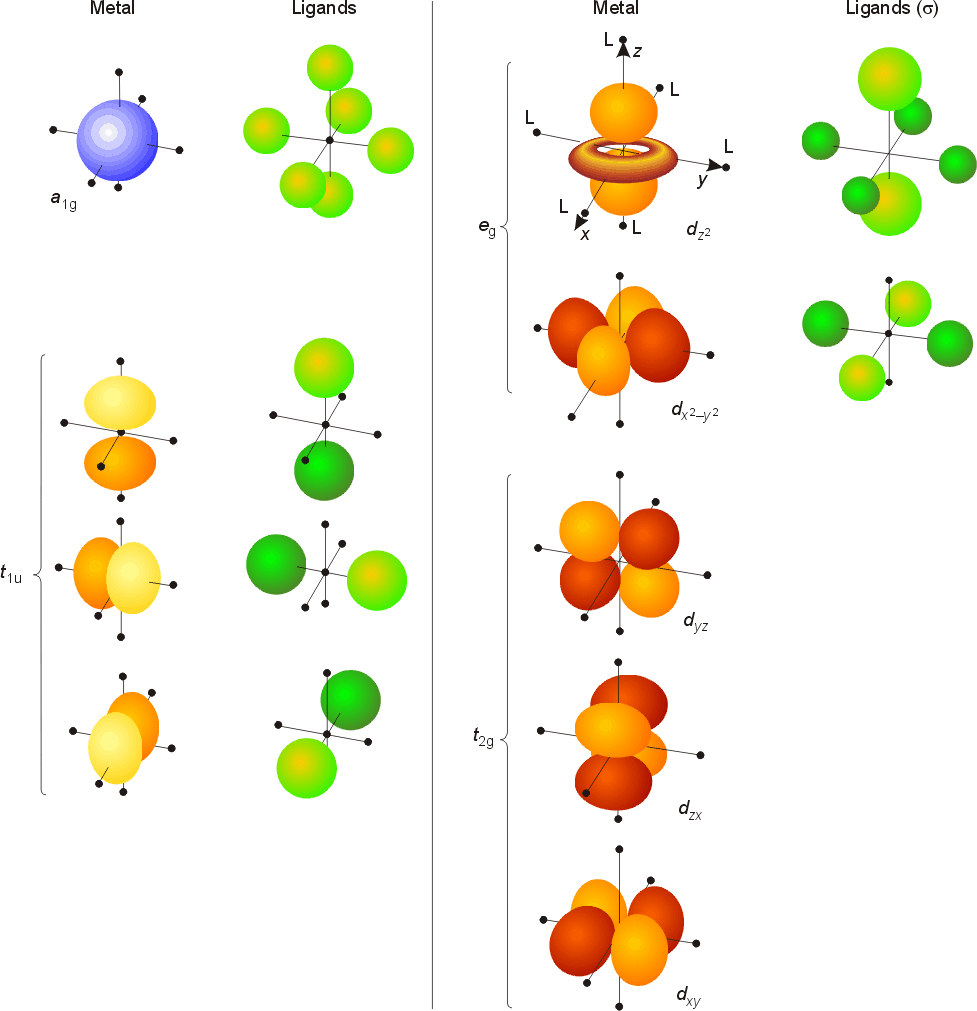
D Metal Complexes

What Is Shape Of D Orbital Designated As Dxy Dyz Dxz Dx2 Y2 Dz2 Chemistry Structure Of Atom Meritnation Com

Development Of Quantum Theory Chemistry
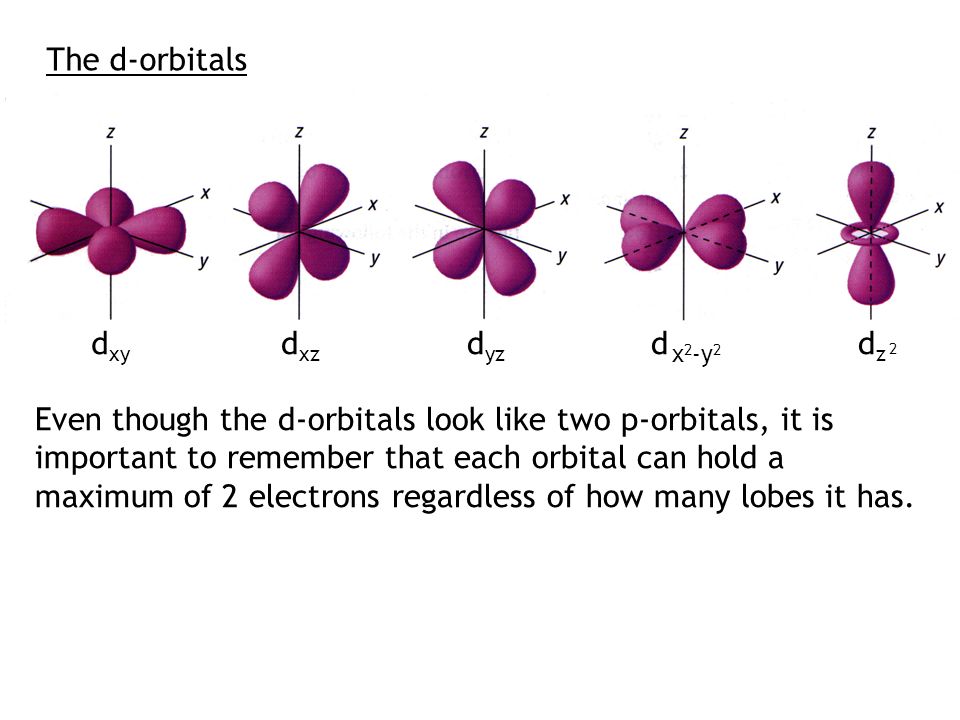
Within An Energy Level N 1 2 3 4 There Exists N Types Of Orbitals And N 2 Sublevels Norbital Types One S Orbital Three P Orbitals One S Orbital Ppt Download

Draw The Shape Of Dz Orbital Brainly In
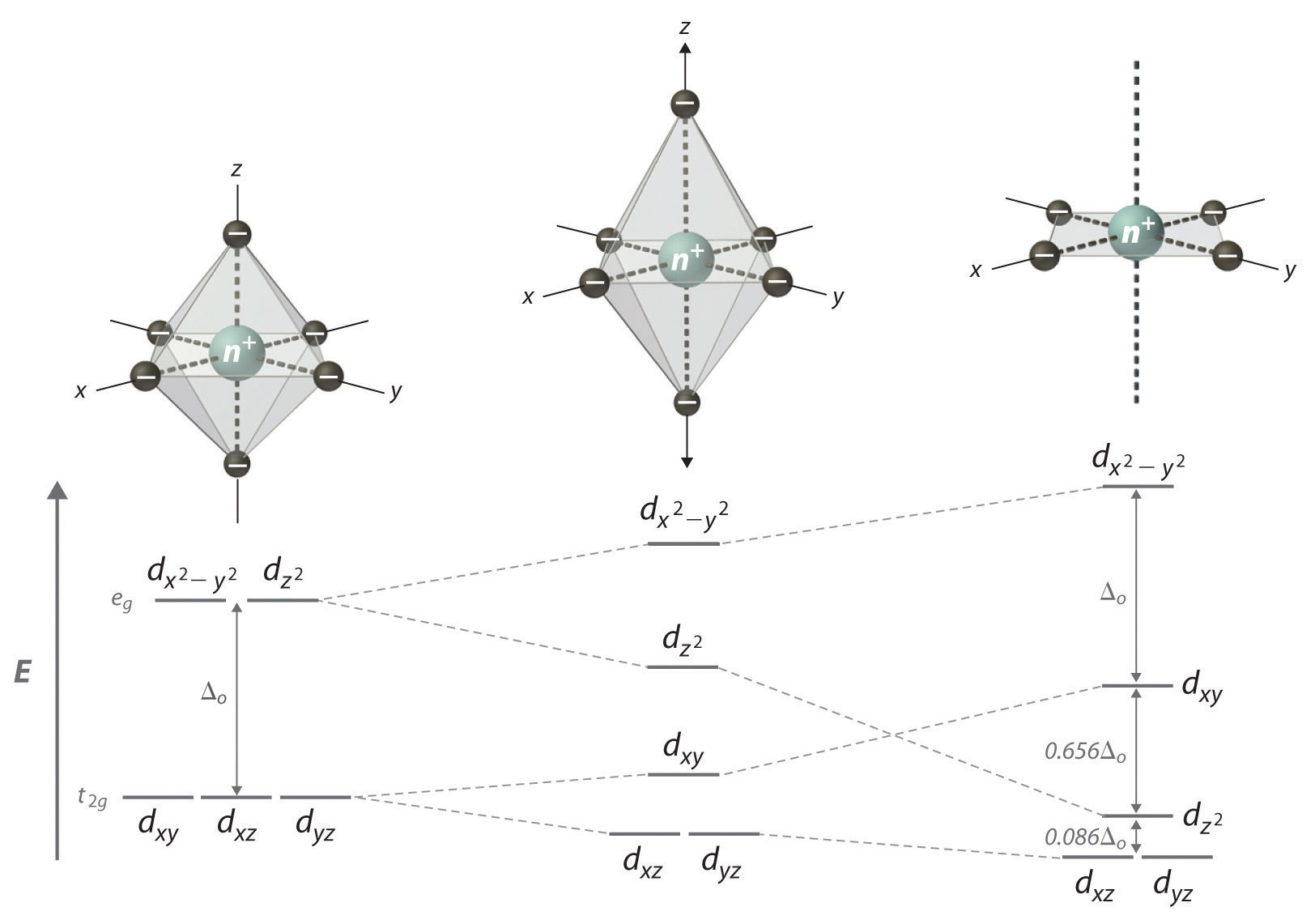
What Is The Significance Of The 3d X 2 Y 2 Atomic Orbital Socratic
Opencommons Uconn Edu Cgi Viewcontent Cgi Article 1047 Context Chem Educ

A R Donation From The Filled Ligand P X Orbital To Vacant Metal 5d X Download Scientific Diagram
A Energy Levels

The Dx 2 Y 2 And Dz 2 Orbitals Are Directed Along A Set Of Mutually Perpendicular X Y And Z Axis And Are Called Eg Orbitals If True Enter 1 Else Enter 0

Schematic Diagram Of Cu 3 D X 2 Y 2 Orbitals And O 2 P S Download Scientific Diagram

Chapter 2 Section 2 4 Open Inorganic Chemistry
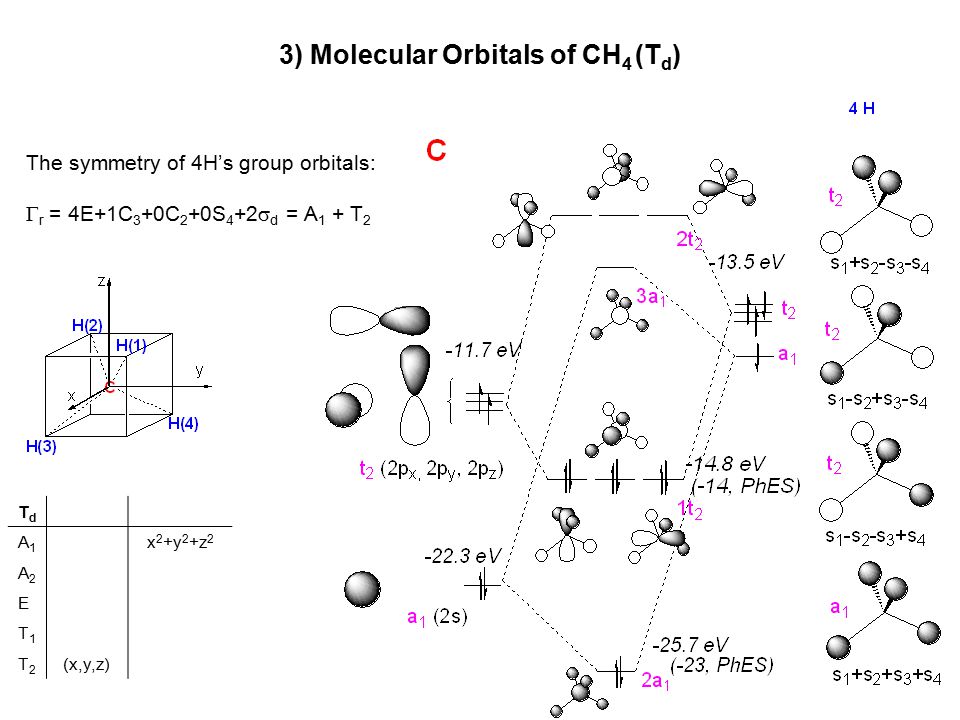
Lecture 17 Molecular Orbital Theory 1 Molecular Orbitals Of Ah X X 3 4 6 Mo Diagrams Can Be Used On A Qualitative Basis To Understand The Shape Ppt Download




